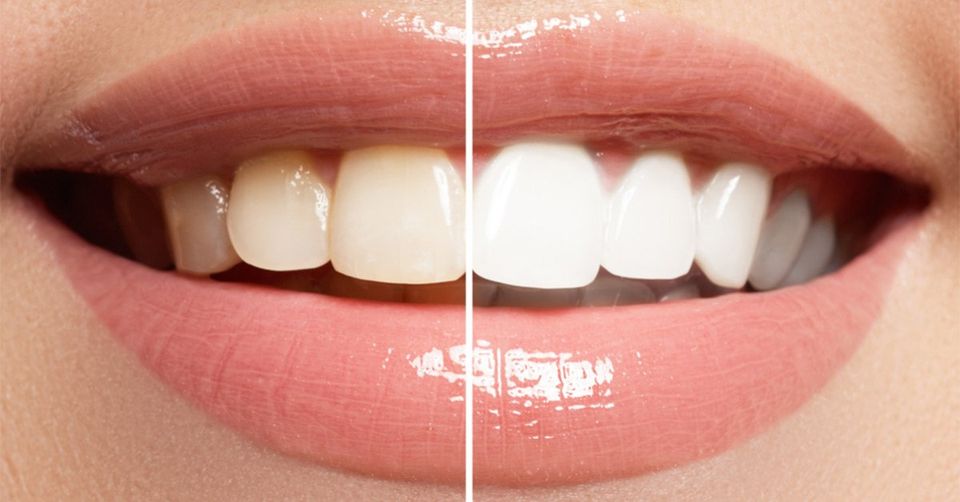
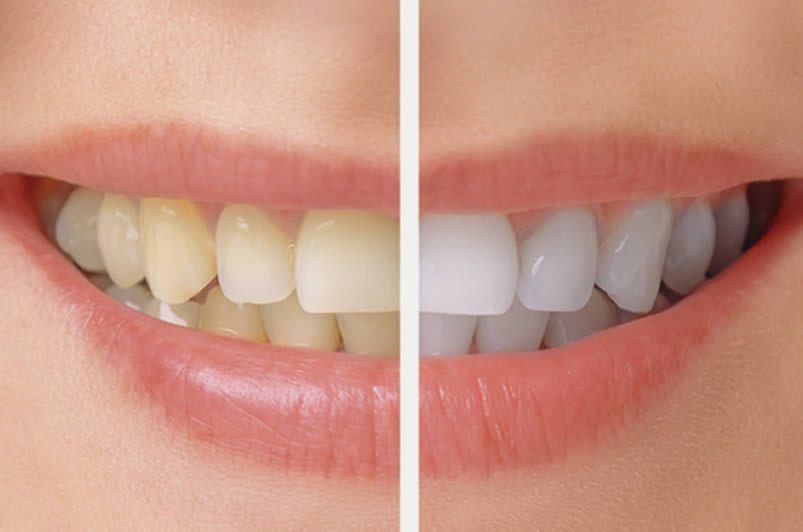
Tooth whitening (termed tooth bleaching when utilizing bleach), is either restoration of natural tooth shade or whitening beyond natural tooth shade, depending on the definition used. Restoration of the underlying, natural tooth shade is possible by simply removing surface (extrinsic) stains (e.g. from tea, coffee, red wine and tobacco) and calculus (tartar). This is achieved by having the teeth cleaned by a dental professional (commonly termed “scale and polish”, see debridement and polishing), or at home by various oral hygiene methods. Calculus is difficult to remove without a professional clean. To whiten the natural tooth shade, bleaching is required. It is a common procedure in cosmetic dentistry, and a number of different techniques are used by dental professionals. Many different products are also marketed for home use. Techniques include bleaching strips, bleaching pen, bleaching gel, and laser tooth whitening. Bleaching methods generally use carbamide peroxide or hydrogen peroxide. There are claims that carbamide peroxide is less effective than hydrogen peroxide but also has fewer side effects. Common side effects of bleaching have increased the sensitivity of the teeth and irritation of the gums. Occasionally individuals develop an unhealthy obsession with tooth whitening akin to body dysmorphic disorder, termed “bleachorexia”.
Whitening methods include in-office bleaching (applied by a dental professional), and treatments which the individual carries out at home (either supplied by a dental professional or available over the counter). In some countries, non dental professionals also carry out tooth whitening procedures for consumers. Bleaching solutions generally contain hydrogen peroxide or carbamide peroxide, which bleaches the tooth enamel to change its color. Off-the-shelf products typically rely on a carbamide peroxide solution varying in concentration from 10% to 44%. Bleaching solutions may be applied directly to the teeth, embedded in a plastic strip that is placed on the teeth or uses a gel held in place by a mouth guard. Carbamide peroxide reacts with water to form hydrogen peroxide. Carbamide peroxide has about a third of the strength of hydrogen peroxide. This means that a 15% solution of carbamide peroxide is the rough equivalent of a 5% solution of hydrogen peroxide. The peroxide oxidizing agent penetrates the porosities in the rod-like crystal structure of enamel and breaks down stain deposits in the dentin.
Source: Wikipedia